Understanding CAR T-Cells
CAR T-Cells are genetically engineered immune cells designed to target and destroy cancer cells. By reprogramming a patient’s T cells to recognize specific cancer markers, these therapies offer highly personalized and effective treatment. CAR T cell therapies are revolutionizing cancer care by providing long-lasting remissions and hope for previously untreatable cancers.
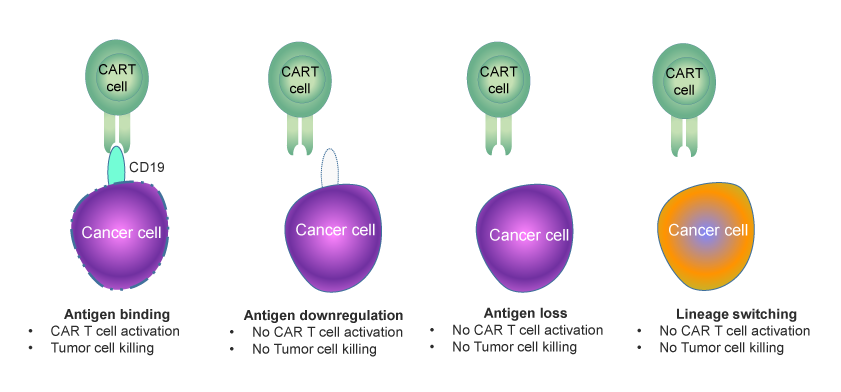
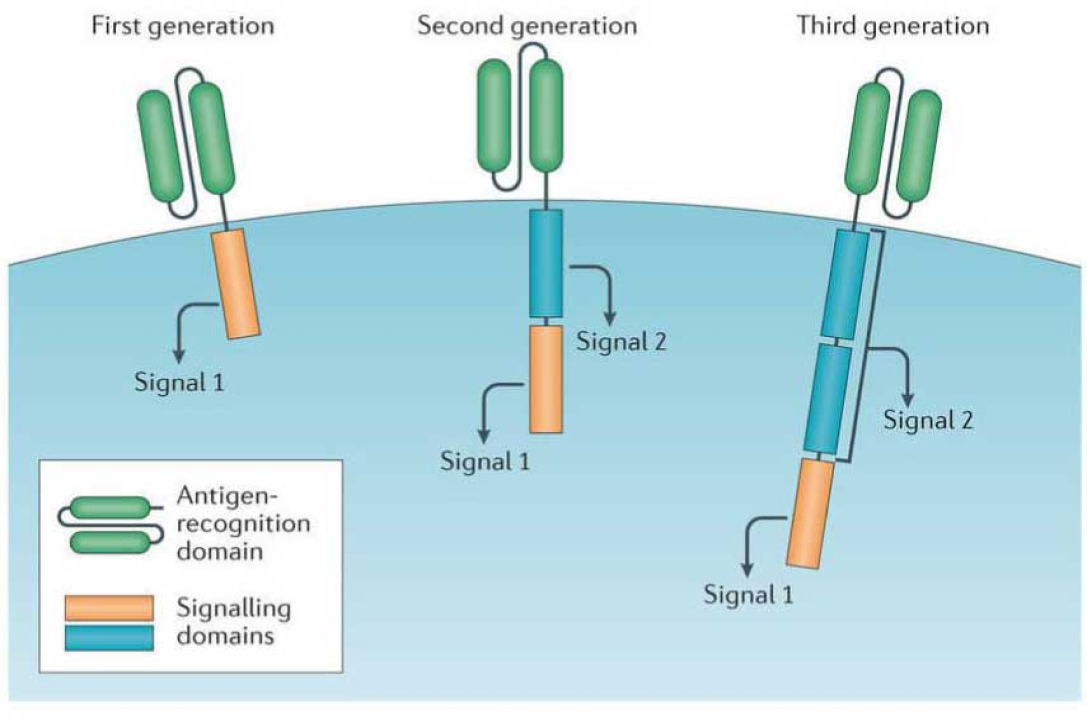
Over the years, significant progress has been made in optimizing CAR designs, improving their efficacy and safety. However, certain critical challenges remain unaddressed. Issues such as antigen escape-mediated relapse, where cancer cells mutate to evade detection, and loss of CAR T cell persistence significantly impact treatment outcomes. These challenges result in therapy failure in approximately 50% of patients, underscoring the need for ongoing research and innovation to enhance durability, precision, and effectiveness. New advancements in dual-targeting CARs and strategies to improve T cell fitness are among the promising approaches being explored to overcome these barriers.
There are two major challenges faced by first and second-generation CAR T cell therapies: